The beryllium alloy or copper alloy, or even Be-Cu alloy is a material well known in Formula one. It also is a known industry despite the difficulty of separating pure beryllium compounds from the principal minerals and by the difficulty of reduction of the beryllium compounds [1,2]. From 1932 the United States already produced Be-Cu with 12 to 13 per cent beryllium. Alloys with 2 to 2.25 percent of beryllium already were commercialized in the form of sheet, rod, tube and wire [3]. From this period until nowadays, many alloys of beryllium with copper, nickel, aluminum and iron are available in quantities and at prices that permit them to compete with other engineering materials.
Chemical properties
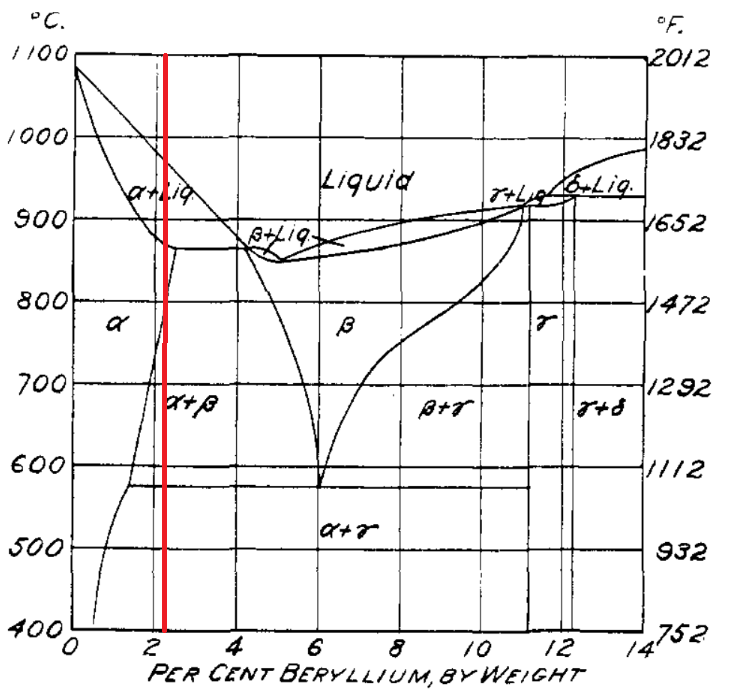
The alpha phase has the face-centered cubic lattice of copper proportionally to the beryllium content. Alloys in this range are malleable and ductile. The pure beta phase and pure gamma phase are extremely hard and brittle. However at very low beryllium percentage, there is little gamma present and in coarse particles. In this phase alloys are nearly as malleable and ductile as the pure alpha phase. In general, under equilibrium conditions the ductility decreases and the hardness increases in proportion to the percentage of gamma in the structure [4]. When the proportion and distribution of gamma is fixed by carefully controlled heat treatments, the alloys have remarkable mechanical properties [4]. The beta plus alpha phase is the balance between harder and more brittle in the cold and the alpha phase weakness at hot-working temperatures. The best Be-Cu setup is the beryllium content of 2.0 to 2.25 percent. Quenching from 800-820° C. produces a ductile, malleable structure which can be cold-worked by manufacturing processes as rolling, drawing, swaging, pressing, and the like. At temperatures below about 775° and above 575° C the presence of the beta phase in the structure permits the alloy to be hot-worked. After all of the forming operations are completed, pieces made from beryllium-copper can be hardened and strengthened by simply heating them for a certain period of time at a comparatively low temperature [4].
Applications
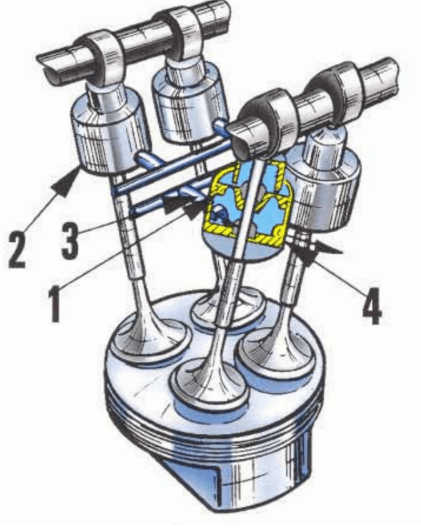
The BeCu alloy has several applications in the automotive field.
- Seat inserts for titanium valves
- Bearings and bushes
- Electronic components
- Valve pins
- Electromagnetic shielding gaskets
- Fasteners
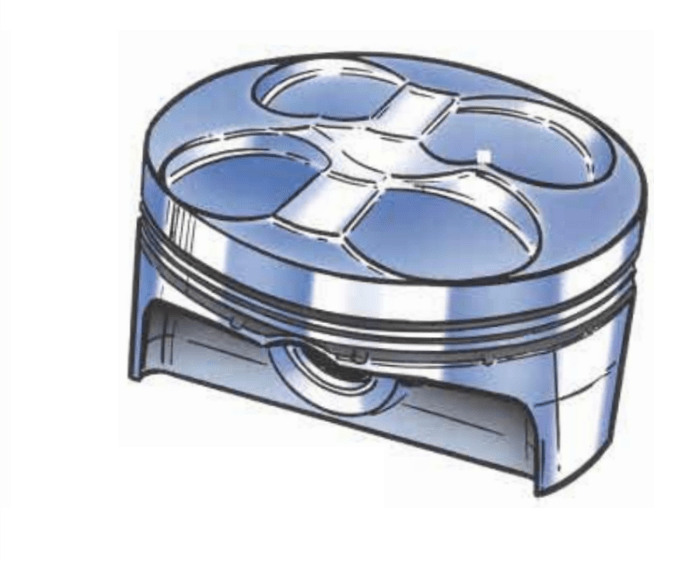
- Pistons.
Beryllium copper valve seats and guides are used in high performance four-stroke engines with coated titanium valves. It dissipates heat seven times faster than powdered steel or iron seats and guides. The softer BeCu reduces valve wear and increases valve life. The manufacture of BeCu springs have the corrosion resistance of copper plus high resistance to fatigue, high resilience and low hysteresis loss. The good wear resistance is advantageous in precision bearings, bushings, ball cages, adjustable-pitch propeller hub cones, gears, and sliding contacts. The most known BeCu alloy is the C17200 (alloy 25). It is a beryllium-copper alloy which offers a broad range of attractive performance characteristics. These include good conductivity and corrosion resistance. In addition, C17200 is non-magnetic and its features are insensitive to machining and surface abrasion. Once fully heat-treated, no additional treatments are required. The combination of galling resistance, high hardness and low friction results in excellent wear resistance in components such as bearings, bushes and even pistons. The corrosion resistance of the product is similar to pure copper. BeCu alloys have the highest strength of any copper alloy combined with electrical conductivity. In addition their machinability weldability processes are all rated as good.
Beryllium banning
Although BeCu alloys are permitted by FIA F1 Regulations [1], that limits the percentage of Be to 2,5% by weight for car construction and 2,75% for powertrain, this material was already banned two decades ago. Between 1998 and 2001 the battle for the championship was between McLaren and Ferrari. Both teams ran some races behind the understanding of each comma of the rule book as much as it was on the track [1]. The climax of these races became really high when Ferrari realised that the McLaren Mercedes engine was able to reach as high rpm in addition to a longer piston stroke, thus more horsepower. This is a kind of antagonic parameter (stroke and high rpm) if a proper material is not being used. This material, beryllium alloys. Hence, with a longer stroke, Mercedes engine reaches the same revolutions as Ferrari do owing to the elastic properties of beryllium, that between 2,0 and 2,5 % of it exhibits a ductile and malleable structure.
Beryllium alloys can also be used to produce either the pistons or cylinder linings as an alloy with aluminium. On October 6th, 1999 the FIA decided to ban beryllium. The teams must remove it from their engines until the end of the season. In that period only Mercedes and Peugeot were using it [1]. Finally, beryllium was banned entirely for 2001, and Mclaren realised that the power it had in 2001 was no more than the power it had in 1998 [1]. The beryllium banning were the issues of cost and safety. It is expensive to mine and difficult to work with [3,6]. The other is safety, and in this case, beryllium is poisonous and carcinogenic. The beryllium alloys has already been a research theme since the 1980 decade. Population-based surveys for sensitization to beryllium and chronic beryllium disease (CBD) have been conducted since 1987. CBD is a disease in sensitized individuals that primarily affects the lungs and is characterized by non-caseating granulomas and interstitial infiltrates, leading to fibrosis. Symptoms are nonspecific and include cough, shortness of breath, fatigue, and night sweats. Among industry staff, the highest levels of beryllium were just found in rod and wire production [5,6]. Therefore the process of C17200 pistons requires a high control of the machining process due to its carcinogenic effect [1,5,6]. Although once the piston is manufactured, it has no danger, this material was banned. Eventually this did not control f1 team costs. Teams had to research another elastic material. A mixture of boralyn and aluminium was proposed, but proved even more expensive [4]. Understanding that opposition over individual parts of the engine regulations to control costs was resulting in tiring inspections and controversial bannings, FIA chose to implement the regulations where engine specifications are frozen for a fixed number of years. Whether the FIA was political or biased in its decision to ban high percentage of BeCu alloys during that season it is never known. However it supports this decision in a very good argument, labour safety.
References
- FIA F1 technical regulations 2021.
- https://www.racefans.net/2007/02/08/banned-beryllium/
- https://mineralseducationcoalition.org/minerals-database/beryllium/
- Silliman F., Horace. Beryllium-Copper Alloys. The American Brass Company, Waterbury, Conn, 1936;
- Schuler, C. R., Kent, M. S., Deubner, D. C., Berakis, M. T., McCawley, M., [1] Henneberger, P. K., Kreiss, K. Sensitization and chronic beryllium disease at a primary manufacturing facility, part 3: exposure–response among short-term workers. Scand J Work Environ Health. 2012;38(3):270–281. doi:10.5271/sjweh.3192.
- Process-related risk of beryllium sensitization and disease in a copper-beryllium alloy facility. American Journal of Industrial Medicine, 47(3), 195–205. doi:10.1002/ajim.20140.