The aluminum is a metal with very interesting characteristics, for instance its low density, high fluidity, low melting point and good corrosion resistance. In addition it can be easely alloyed with other materials. However, neither all aluminum alloys are usefull for automotive applications. Therefore this article proposes a summary about the main aluminum alloys which are commonly used in automotive industry.
2xxx series
The two series aluminum alloys are very common in automotive industry, because its mechanical properties. In fact they are similar to low carbon steels in terms of strength. The main chemical composition is aluminum and Cooper or aluminum, Cooper and magnesium. Although very strong, this grade is also subjected to intergranular corrosion due to the precipitates.
The most commonly used 2xxx series aluminum alloy is the 2024, usually with T3 heat treatment, which means solution heat treated, cold worked and naturally aged to a substantially stable condition. There are several 2xxx series aluminum alloys , but for automotive applications the main ones are the 2024 and the 2099.
The difference between these two is the alloy element, while 2024 are alloyed with Cooper or Cooper together with magnesium, the 2099 is alloyed with Cooper and lithium, which in this case they provide an even higher mechanical strength comparable 7xxx series aluminum alloys.
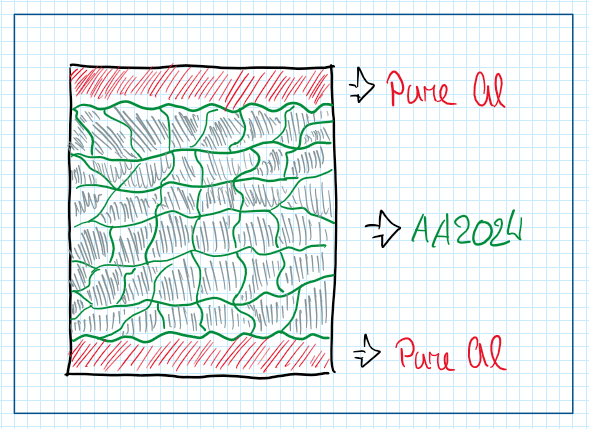
The main problem with the 2024 aluminum alloys is the intergranular corrosion. To avoid this phenomena they are usually developed in a sandwich structure. This is a strategy which the structure composed by 2024 aluminum alloy sandwiched by layers of pure aluminum. The objective is to avoid intergranular corrosion, because even though there is a different potential between the layers, the pure aluminum is exposed to the air and the other lawyer of it is exposed to the aluminum 2024 alloy. In this way the pure aluminum lawyer acts as a protection and reduces the intergranular corrosion.
The 2099 aluminum alloy can provide a higher mechanical property comparable to the 7075 and the 7055 aluminum alloys. The main reason is the increase of the amount of copper and the lithium. However this alloy exhibits a fatigue resistance similar or equal to the other 2xxx series alloys. The 2099 aluminum alloy applications are concentrated in the aircraft construction, aerospace industry and high performance vehicles.
7xxx series
Another kind of aluminum alloy applied in automotive industry is the 7th series. These are characterized by their hi strange, similar to structural steel. these good mechanical properties kames from its alloying elements which are chromium , manganese and zirconium.
the adoption of those elements results in the production coma after temper, of dipersoids which in the end forms the intermetallics. This one are formed at very high temperature, are not solubilized , precipitate at grain boundaries, enhance the grain stability and prevents the grain coarsening. In fact the formation of dispersoids represents a finer grain structure , because the particles of 1 substance dispersed on the another are very well divided.
Even though those characteristics are very attractive, the seven series aluminum alloys are usually exposed to stress corrosion cracking and develops a low fatigue resistance do it to the same reason as they are strong. There’s reason is the precipitate MgZn2 which is formed in the end of the Asian process. The sequence of this process is the solid solution strengthening, which forms the guinier preston zones, the quenching and finally, the aging that transforms the dispersoids on intermetallics.
7075 Ergal
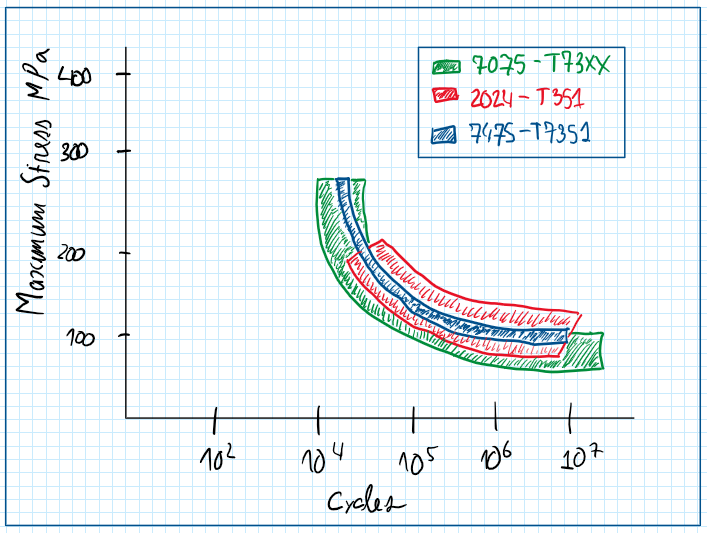
The 7075 aluminum alloy , also called ergal , it’s a variation from the seven series with the addiction of copper. Its main chemical composition usually includes aluminum, zirconium magnesium in chromium. As all 7th grade aluminum alloys also have a problem with corrosion, thus the heat treatment is the temper T6. However it is possible to find some variations about the T6 and the T7 temper processes. These have specifics objectives and usually are applied in well defined purposes. The objective of these heat treatments is to improve the corrosion resistance. In fact any corrosion resistance improvement is followed by a decreasing of the strength. This is useful to make this kind of aluminum alloys more resistance to stress corrosion cracking.
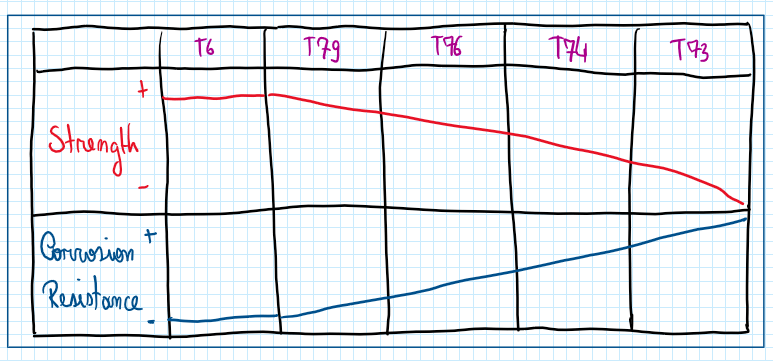
As in all aluminum alloys, if more alloying elements are included more the corrosion resistance reduce. For this reason for seven series there are different aging treatments , which are usually variations from T6 and T7. However all of them represents a tradeoff between strength and cohesion resistance. If the corrosion resistance is improved by those aging treatments these trends reduces.
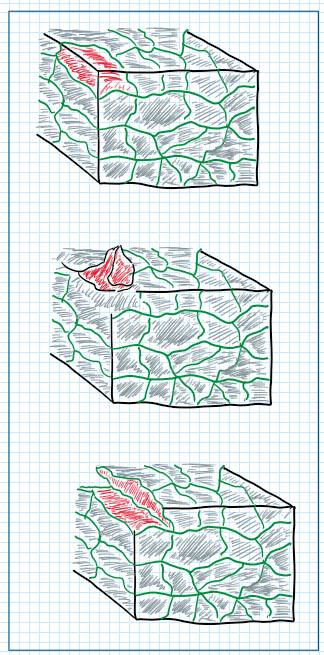
This tradeoff is accepted because the seven series is exposed to the stress corrosion cracking. This cohesion process begins with integral conversion but due to the nature of this process the cohesion propagates over the entire grain boundary. As in seven series aluminum alloys the grain boundaries are long the result is the detaching of slashes of the material , after phenomena called filiation. Therefore those heat treatments for instance T6 and T73 prepare the material for avoidance of exfoliation and stress corrosion cracking, respectively.
7055
Is considered an evolution of the 70 75 aluminum alloy , due to the increased amount of zirconium and zinc. In fact this is a variation of ergo with the same chemical components (aluminum , my mission, Cooper and zinc) . Hence this material is a has a better yield strength and exfoliation resistance relative to the 7075. As a result a smaller cross-sectional area can be used which means a lower weight. These characteristics make 7055 more interesting for aircraft industry.
Al-Li alloys
Use it specifically for automotive bodies the aluminum lithium alloys exhibits several characteristics which justifies their use in automobiles. Its low density is about 8 in 10% lower then the other aluminum alloys which combined it with its high young modulus (about 8 gigapascal), provides a very interesting stress/density relation. Even though, the aluminum lithium alloys exhibit a lower ductility and toughness and cost 2-3 times higher then 7055 aluminum alloys.
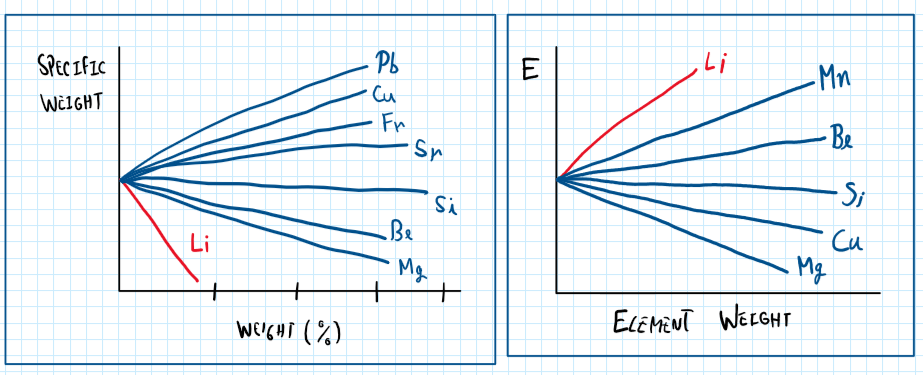
Due to the increase amount of lithium, these alloys are respective to the 8 series. According to the Figure above , lithium additions improves the mechanical properties of the aluminum alloy while the very low specific weight of lifting do not increase significantly the material weight. However , there is a limit which lithium can be used as in alloying element coma until 4% of litter periods. This means that each 1% of lithium results in a weight reduction of 3% and an increase of 6% of young models.
There are some problems in the use of lithium, as its high cost and difficult handling during manufacturing. In fact, it is fast oxidized, segregates very fast during casting when sodium and potassium impurities are found, they form a low melting point compounds. In addition aluminum-lithium binary alloys do not exhibit interesting mechanical properties. This is the reason why there are not Al alloys without other alloy elements together with aluminum and lithium.
The main temper is the T3 and T8. However it is common the application of a slight plastic deformation ( 2 to 6% ) after quenching . This creates dislocations whose enhance the formation of precipitates during aging treatment.
Al-Sc alloys
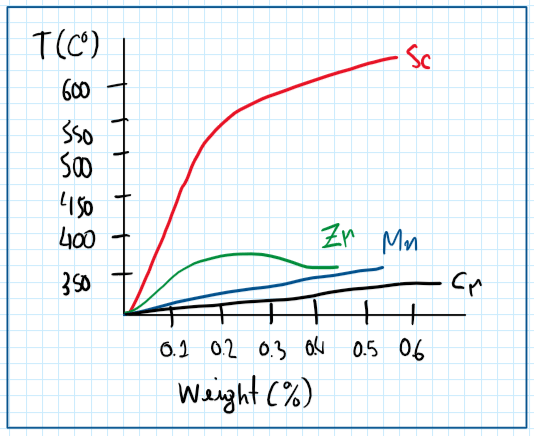
The objective of adding scandium to an aluminum alloy is to increase the mechanical properties. This is obtained because scandium form a supersaturated solid solution after solution heat treatment, then after the temper it is formed a secondary phase Al3Sc. These dispersoids are highly coherent and provides a fine grain structure, thus an increased strength. In addition, as can be seen by the graph, scandium has a very high thermal stability, which motivates a high recrystallization temperature.
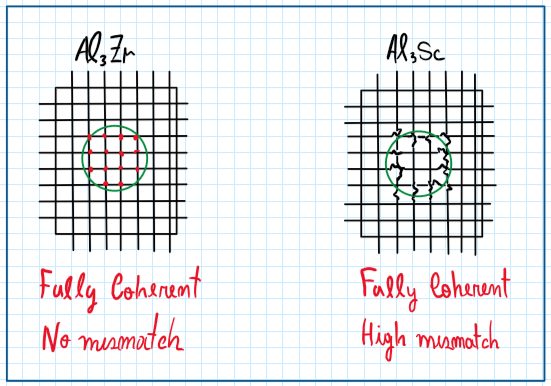
The effect of scandium dispersoids is a significant mismatch on the lattice. When compared to the mismatch caused by Al3Zr, there is a very low distortion of the lattice, 1.2% against 0.8 from Al3Sc. Hence, aluminum-scandium alloys are on the limit of a coherent structure being sometimes considered semi-coherent.
Therefore aluminum scandium alloys exhibits an improved strength, recrystallization temperature , a reduced grain size and an improved resistance to cracking (good weldability). In addition this alloy is capable to hold its grain size even after welding.
Casting Al alloys
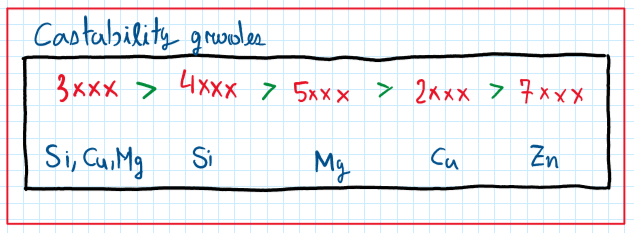
They aluminum as a material has several advantages, one of them is the low melting point. Together with high fluidity at high temperatures they’re aluminum became I material which is very recommended for casting processes. This allows great variety of shapes which the molds can assume to manufacture parts and components which previously are considered not feasible. Another advantage of the aluminum alloys for casting is the final surface roughness after casting, which not require so many post processes and finishing. However the casting process requires some controls to avoid the shrinkage phenomena ( 3.5 to 8.5% ) which for aluminum is a very high number.
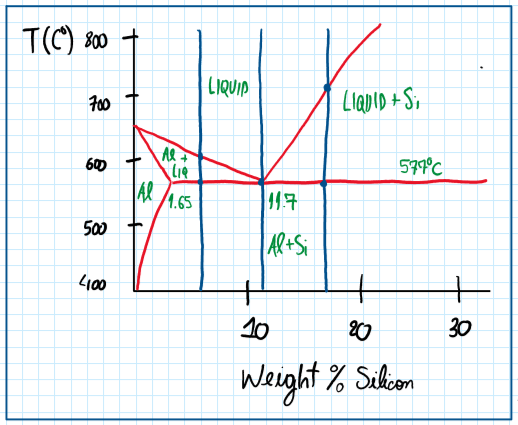
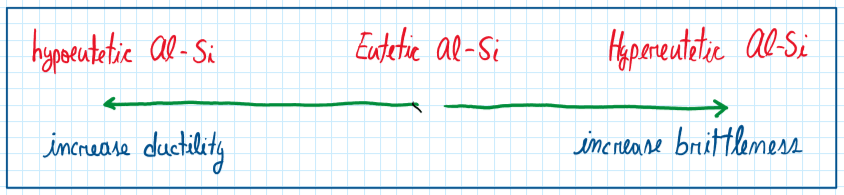
As can be seen the better aluminum alloys forecasting are alloyed with silicon. However, the amount of silicon aided must be controlled to avoid the microstructure coarsening. In general just 10% of silicon is enough to generate a composition around 12.7%. in this case, the structure produced is composed by large plates or needles of silicone. This structure is called hypeutetic and is characterized by a very high brittleness.
Around 10% of silicon it is possible to obtain and structure called eutetic . Although this is the most used configuration, it is brittle due to the sharp lamelae of silicone which concentrates stress. Hence, this configuration requires modification , but it provides a low melting point and blue good fluidity. The hypoeutetic aluminum silicon alloy provides great grains of aluminum and sharp grains of silicon. The main properties of the aluminum silicon alloys are good castability and fluidity ( when eutetic), high corrosion resistance, good weldability, low solidification shrinkage and, in some hypeutetic alloys, the machine process are more difficult.
All aluminum silicon alloys are treated by a process called fluxes. This is a process which solid particles of an element is poured on the cast aluminum, thus according with which element is added there is a flux type. The main flux for aluminum silicon alloys is performed by the addition of alkali fluorides.
The main objective of the fluxes is to transform a needle or lamella like structure to a fibrous one. In general this is provided by strontium and sodium fluorides and they also improve the grain refinement. Consequently, the microstructure is more resistant to hot tearing, decreasing its porosity and increases it mass feeding. However, as high silicon percentage is, higher will be the amount of the modifying agent. The usual strontium concentration is about 0.02%.
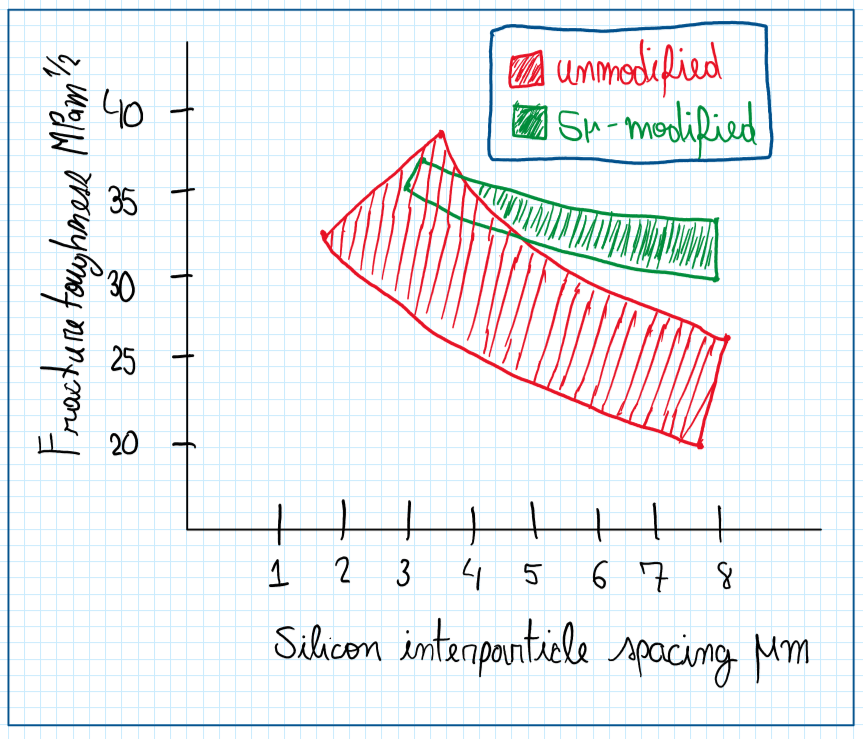
The use of strontium fluorides on aluminum silicon alloys improves the toughness if the size of the silicon particles increases. In other words, even with a not so fine structure, the mechanical properties are improved. In fact, a better grain refinement provides a higher tensile strength, elongation, hardness and fracture toughness. However casting aluminum alloys usually have lower mechanical properties relative to the wrought aluminum alloys. Hence the treatment with fluorides is a alternative process to obtain a better microstructure and an improved mechanical properties.
The flux process is a pouring of solid particles elements in the cast aluminum. If these one made by strontium are substituted by sodium, it is possible to restrict the growth of silicon particles by its segregation on silicon plates periphery and prohibiting growth. This is called “modification” mechanism and it still is a controversial process because it depends on the amount of phosphorus. The increase of silicone particles though reduces the mechanical properties. Therefore the flux processes depends of the element added. In case of sodium, it produces a better result than strontium, phosphorus reduces the performance of the flux process while magnesium motivates it. The specific amount of modifying element added, which depends of the amount of silicon, and the higher solidification rates assist the modification process.
The modification process depends of the element added and its quantity, if this overcome certain values the process is called overmodification . For instance when the amount of sodium exceed 0.018 – 0.020%, this creates a second phase AlSiNa that causes the coarsening of the grains which decreases the mechanical properties.
Al-Si-Cu, Al-Si and Al-Cu alloys
There are some specific aluminum alloys which are indicated for casting, some of them are the aluminum silicon copper and aluminum silicone magnesium. This kind of casting aluminum alloys are usually applied for cylinder heads and blocs of automotive internal combustion engines. The use of copper and silicon allows an increase of the machineability and strength but reduces the castability, ductility and corrosion resistance of aluminum alloys with copper. The silicon improves the capability to be used in high pressure die cast process. The sand permanent mode casting are usually adopted to alloys with lower amount of silicon and higher amount of copper.
Another type of aluminum alloy used for casting is the AlCu. Due to the Cu as alloying element this variation exhibits a very high strength and hardness at temperatures above 250oC. In addition this is also obtained due to the precipitation hardening together with dispersion hardening, which is obtained by intermetallic compounds. Although the strength is higher than all other casting aluminum alloys, it does not overcome the wrought aluminum alloys. This is usually applied in the aerospace industry and some high performance automotive parts.
References
- Ashby, F. Michael. Materials Selection in Mechanical Design. 3rd Edition, Elsevier, London, 2005;
- Davies, Geoffrey. Materials for Automotive Bodies. 2nd Edition, Elsevier, 2012. ISBN: 978-0-08-096979-4, DOI: 10.1016/C2010-0-66319-X.